Feasibility of Using Non-Chemical Methods for Control of the European Chafer (Rhizotrogus majalis) in Turfgrass (2003)
Carolyn Teasdale and Deborah Henderson, E.S. Cropconsult Ltd., Vancouver, British Columbia
Summary
European Chafer is an emerging, recently introduced pest to British Columbia. It was first detected in New Westminster. Since many municipalities and residents are proponents of restricting chemical pesticide use in urban environments, it is prudent to examine alternative control methods. This study examines several biological controls (nematodes, fungi, and bacteria) for European chafer.
Results
- The nematode Heterorhabditida bacteriophora gave significant control of second-instar chafer larvae.
- Insecticide use is expected to be more economical than biological control options, since high rates of nematodes are needed for control
- The bacterial and fungal agents tested did not control the chafer significantly under the conditions of this study.
Back to Top
Developing field monitoring techniques and determining the potential of Steinernema feltiae an entomopathogenic nematode, to control European Craneflies in turfgrass. ( Final report - 2003)
Dr. M. Dogterom , E.S. Cropconsult Ltd, 3041 West 33rd Avenue, Vancouver, BC V6N 2G6
1. Population of Craneflies 2001-2003.
Cranefly populations were not abundant, and were not a problem for the Turf Grass Industry as surveyed in fall 2003. Between 2002-2003 adult craneflies/larvae-damage was in localized areas and not extensive. High populations of cranefly in the mid-1990's seem to have fallen since that time. Often when a new species invades a new area, it takes a few years for it to be controlled by natural factors (i.e. predators, parasitoids, epizootics) however with some pests, this doesn't happen. Fortunately with cranefly, it seems that the population explosion of the mid 90's has declined.
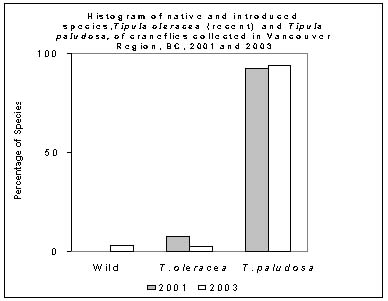
2. New European Cranefly species as a proportion of the general Cranefly population.
The new species of cranefly T. oleracea were present in small numbers over the three years that craneflies were sampled. Tipula oleracea was less than 10% of the population (2.5% in 2003, and 78.8% in 2001). The new species may become just another resident species not causing significant new problems.
3. Monitoring Techniques for Adult Cranefly
In 2001-3 we investigated and developed various techniques to collect, examine and handle cranefly adults. We examined sticky traps, counts and sweep techniques for monitoring adults. Most cranefly were observed and counted during visual counts of flying adults. Sticky cards appeared less useful since they were destroyed by vertebrates and appeared more useful when abundance was high rather than low. Craneflies appeared to be able to avoid sweep nets. 1 min visual counts were most effective in determining abundance of adults.
4. Monitoring Techniques for Larval Cranefly
We examined turf for cranefly larvae throughout the year. In late fall and early spring cranefly larvae were too small to be found. Cranefly larvae in May are 2-4 cm in length and are easily found in turf. However this method is labor intensive. A more efficient method is to submerge turf in water to completely submerge the roots but not the grass surface. Larvae move to the surface above the water and into the surface of the grass where they can be counted. An indicator of abundance can be the ease by which larvae can be found. When larvae density is more than 20/sq foot larvae are easily found and at this density turf destruction is notable.
5. Efficacy Studies with nematodes for control of Cranefly larvae
Immature larvae
We tested immature larvae under laboratory and terrarium conditions. The petri dish trial in 2002 indicated that nematodes are effective against newly hatched larvae. However, these results are preliminary since only a small number of cranefly larvae were tested. The 2003 trial was unsuccessful in emerging any larvae for the trial. The outdoor terrarium trial (2002) was not successful since no craneflies were found in either control or nematode plots.
Mature larvae
We tested mature larvae under terrarium and field conditions. The aquarium trial (2002-2003) indicates that nematode treatment had an effect on the survivability of the cranefly larvae. More studies are needed to confirm these data since overall survivability of both treated and control plots was less than 10%. The field trial (2001-2002) was inconclusive since cranefly densities were low in abundance with less than 3 cranefly larvae per square foot.
Assessment of cranefly populations is recommended as an ongoing protocol for turf grass managers. The fall flight can be used as a predictor of spring larval populations and potential damage to turf. Follow-up examinations for larva in turf during May/June would confirm whether populations remain stable, are in decline or are on the rise.
Protocol for Cranefly monitoring by turf grass managers:
Craneflies can be assessed qualitatively as None, Few, Moderate, or Abundant. 'Few' = occasional sighting; Moderate = hundreds , Abundant = thousands.
1. Record date and assess adult cranefly flight in the fall (Sept-October). After the first day that craneflies are observed, record daily numbers until flight numbers decline. Five '1 minute counts' while walking slowly across the turf in selected areas will provide good abundance data. The recommended time to complete counts is between 9am-noon since temperatures are rarely warm enough for emergence and flight prior to this time.
2. Record larval cranefly numbers in the soil (May-June). The recommended sod size is 6" x 6". It is recommended that the sod is broken up into smaller pieces so larvae can be found and counted. Counts of 0-50 are acceptable numbers unless sod is not well established. Counts of more than 50 indicate a cranefly problem and sod damage. (for more details on management go to click here
3. Compare yearly records and determine 3-5 year trends in order to assess whether cranefly populations are increasing, decreasing or stable.
Back to Top
Genetic Variation in Resistance of Annual Bluegrass (Poa annua L.) to Snow Molds and Insect Pests and Mechanisms of Resistance.
Progress report presented to the Canadian Turfgrass Research Foundation February 2003
By Dr. Julie Dionne, Co-investigators: Tom Hsiang, Yves Castonguay, Jacques Brodeur
Graduate Students: Louis Simard and Martha Cunningham University of Guelph, Department of Plant Agriculture, Guelph, ON
Context
The turfgrass industry in Canada is conservatively estimated to be worth more than $5 billion, including golf courses, nursery sod production, home lawns and commercial turf, sports fields and municipal parks, as well as supply and equipment industries. Much of the value depends on functional features which are adversely affected by insect and disease pests. With the increasing concern about pesticides, deregistration of some chemistries (e.g. organophosphate and carbamate insecticides) and the potential for complete bans by municipalities, the ability to develop and recommend effective alternatives is critical. While IPM has a proven value in safe and sustainable turf management, there is an ongoing need to add to the array of tools that are available for integration. This is particularly true with respect to improved cultivars resistant to insect and disease pests. The development of low risk alternatives to current pesticides in an IPM context has become critical in the context of the recent Supreme Court decision and impending municipal bans on pesticide use.
This project aims at assessing the genetic potential for snow mold and insect pest tolerance within annual bluegrass and analyzing the molecular basis of its tolerance. Our goal is to provide critical information to guide breeding efforts and management strategies to significantly improve the inherent tolerance of annual bluegrass to biotic stresses.
Summary of results for the first year of research
1. 55 ecotypes of annual bluegrass were collected during spring and summer 2002 and than propagated in the greenhouse. 20 ecotypes were collected from golf course in Québec involved in the insect IPM research project (in collaboration with QTRF). 15 ecotypes were collected from golf courses in Ontario. 20 ecotypes (including the Top twelve quality poa ecotypes) were provided by Dr. David Huff from PennState University.
2. Protocols are currently under development for the inoculation of turfgrass ecotypes in controlled conditions using either infected wheat bran or potato dextrose agar (PDA) infected with the pink snow mold pathogen, Microdochium nivale. Poa annua and creeping bentgrass were inoculated with different methods: i- 6 mm plug removed from PDA with inoculum placed on thatch;, ii- wheat bran colonized by M. nivale (3 rates). Plants were placed in a cold chamber at 4ºC for three months. Disease progression and turf quality was determined weekly and leaf samples are currently cultured on PDA to verify that plant decline is due to colonization by M. nivale. A second replication of this experiment was started in February 2003.
3. We collected black cutworm on golf course during summer 2002. We developed methods for rearing black cutworm and we have now an important population of this insect in our turf laboratory for performing experiment on insect resistance all year long.
4. 30 ecotypes of Poa annua were transplanted into greens late September 2002. Experimental sites are located on golf greens at the Guelph Turfgrass Institute, ON and at the Victoria West Golf Club, Guelph, ON. Plants were allowed to cold acclimate in the field and were inoculated with wheat bran colonized by three strains of Microdochium nivale. Disease incidence and turf quality will be evaluated in the Spring 2003. Dataloggers were placed at both sites to monitor air and soil temperatures.
5. 50 ecotypes of Poa annua were transplanted in pots and cultivated in the greenhouse during fall 2002 and will be used within the next 4 weeks to start experiments on insect resistance since we have now a large number of black cutworm and all poa annua pots available. Two simultaneous experiments will be performed. Damage per ecotype will be evaluated throughout the experiment on a scale of 1 to 9: 1 = extensive damage, and 9 = little or no injury. Insect development and mortality will be recorded throughout the experiment. Larval and pupal weight and length, days to pupation, and adult emergence will be recorded.
Back to Top
Report on European Cranefly (Tipula Oleraceae) Research Project (1998)
Bob Costello, BC Ministry of Agriculture & Food, Abbotsford, BC
Life history
The life cycle of T. oleraceae differs significantly from that of the long established species T. paludosa. A comparison of the two species is shown in Table 1. In British Columbia, T. oleraceae has one generation a year, as it does in central Europe. In southern Europe (Italy an Spain), it has two generations a year.
Table 1: Life history of T. paludosa and T. oleraceae in British Columbia.
| | T. paludosa | T. oleraceae |
| # of generations | one | one |
| eggs | Sept - Oct | May - June |
| larvae | Sept to June | May to Feb |
| pupae | July - Aug | March - April |
Distribution
A survey and discussions with other entomologists in the Pacific Northwest revealed that T. oleraceae was much wider spread than anticipated. It occurs on southern Vancouver Island and throughout the greater Vancouver area as far north as Squamish. One report was received of its' occurrence in northern Oregon and western Washington. This indicates that T. oleraceae has been established in the Pacific Northwest for some years before its' presence was recognized.
Damage
Grass is the preferred host of this leatherjacket species, but European literature suggests it is a very opportunistic feeder. It has been found feeding on Douglas Fir seedlings on Vancouver Island. In late winter the larvae avoid very wet ground, migrating to drier, usually higher, areas. On golf courses this often results in high populations moving to and feeding on the putting greens. Also, when the soil becomes waterlogged and the leatherjackets are driven to the surface, birds will feed on them, resulting in damage to the turf as they scratch and peck.
Control options
Because the two species often co-exist, ideally one pesticide application could be used to control them both. This is most suitably done in October. At that time the larvae of both species are feeding close to the surface and therefore are easier to contact with an insecticide. Also, they are still relatively small so they have not caused significant damage. Additionally, October soil temperatures and moisture levels are sufficiently high to allow parasitic nematodes to be used effectively.
At present, diazinon and dursban are the only insecticides registered for leatherjacket control on turf. We have applied for a Minor Use registration for Sevin XLR as this chemical has been found to be effective for a number of other soil insects. It would be a suitable insecticide for a resistance management program.
Back to Top